Taken jointly, lyophilization is often a important Resource with the preservation of biological samples with quite a few rewards. We goal to attract notice to the big selection of opportunities provided by freeze drying in pre-medical or standard investigation.
While plant-centered foods are the most well-liked freeze-dried products, an array of foods is usually preserved using this technique.
One example is, sugars like sucrose or mannitol, are frequently employed as cryoprotectants to stabilize the structure of proteins through freezing. They prevent the denaturation or aggregation in the API, which especially critical for elaborate molecules like biologics but However they are able to impact the drug merchandise behavior in the course of sublimation and therefore a deep knowledge of how formulation can affect lyophilization process is crucial.
If you need to convey a posh, lyophilized drug merchandise to marketplace, glance no further more compared to industry experts at Particle Sciences.
5% every year during the last 5 years. And this pipeline of lyophilized products will only insert to your established list of lyophilized drugs available now (Table one).
Even though there are a myriad of applications and methods to carry out, the down below is definitely an overall information for the lyophilization process, and many of the ways desired for achievement.
As an authority in cryogenic infrastructures, Demaco makes certain that the liquid nitrogen reaches the freeze dryer at the correct strain from these storage tanks even though in the best possible excellent.
With many years of knowledge inside the pharmaceutical industry, we know precisely what an excellent infrastructure to get a cryogenic freeze dryer needs.
Lyophilization and homogenization of Organic samples increases reproducibility and reduces normal deviation in molecular biology approaches
Lyophilization is a value-effective strategy for Organic specimen preservation but specific tissue-particular reference protocols remain missing. Moreover, info are constrained to the extensive-time period steadiness of proteins and nucleic acids in lyophilized samples.
The subsequent move while in the process is secondary drying. Secondary drying comes about when the last ice crystal has disappeared, along with the merchandise is here then cautiously warmed up from its minimal temperature. This closing dehydration in the item is performed less than a large-temperature vacuum that rids the program of any drinking water that didn't crystallize and was sure to the merchandise’s molecules.
Freezing: transforming The fundamental solution by abstracting warmth to create a point out which is well suited for sublimation drying. When an aqueous application of lyophilization in pharmacy item is cooled, crystal nuclei are shaped. The surrounding h2o is taken up across the nucleation sites, leading to crystals of various dimensions and styles. Freezing velocity, composition of the basic product or service, water articles, viscosity of the liquid along with the existence of non-crystallizing substance are all decisive variables in figuring out the crystal form and dimensions, and in influencing the next sublimation process.
Advertisement cookies are used to supply visitors with related ads and marketing and advertising campaigns. These cookies track readers throughout Internet websites and obtain info to provide personalized ads. Other individuals Some others
The cycle’s parameters, which include freezing fee, shelf temperature, and vacuum stress, are determined dependant on the product or service’s features and security demands. Guided by Good quality by Design and style (QbD) principles, cycle structure is okay-tuned by way of a number of experiments to obtain an In general thriving structure House and array where the lyophilizer parameters can work with achievement.
 Judge Reinhold Then & Now!
Judge Reinhold Then & Now!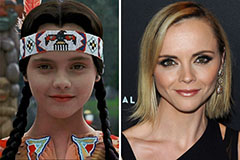 Christina Ricci Then & Now!
Christina Ricci Then & Now! Danica McKellar Then & Now!
Danica McKellar Then & Now! Jane Carrey Then & Now!
Jane Carrey Then & Now! Ricky Schroder Then & Now!
Ricky Schroder Then & Now!